Type 1 Diabetes (T1D) is the second most common chronic illness among children, posing significant health and economic challenges worldwide. This disease can drastically reduce the quality of life and life expectancy of those affected, leading to serious health complications. Moreover, it places a heavy financial burden on society, with the U.S. alone spending about $16 billion annually on healthcare and lost income due to T1D.
Since the 1950s, the number of T1D cases has been on the rise, with current figures at approximately 8.4 million people globally. This number is expected to soar to between 13.5 and 17.4 million by 2040—a near doubling from today’s figures. Particularly high rates of T1D are found in Europe, especially in the Nordic countries and Sardinia, with Finland recording the highest.
Historically, insulin has been the mainstay of T1D treatment, but it does not prevent the disease. Recent research, however, has made groundbreaking strides in this area. In November 2022, the FDA approved a revolutionary therapy that delays the progression of T1D by inducing immune tolerance with a specific treatment. This new therapy can postpone the full onset of diabetes by two years, although it does not prevent the initial autoimmune process or the viral triggers of the disease.
Additionally, promising new research into vaccines and antiviral drugs is underway. A vaccine targeting viruses linked to T1D has successfully completed its initial trial phase, and a recent antiviral drug trial has shown improvements in pancreatic function, which is crucial for diabetes management.
These advances represent a significant leap forward in potentially reducing T1D incidence, which could save millions of lives and significantly cut healthcare costs. If these new treatments and vaccines prove effective on a larger scale, they could not only transform the management of T1D but also inspire similar breakthroughs for other non-communicable diseases. The goal is clear: to develop comprehensive strategies that combine clinical trials, cutting-edge technologies, and innovative research to prevent and manage Type 1 Diabetes more effectively.
The project ENT1DEP is spearheading an innovative approach to understand how infections can lead to non-communicable diseases (NCDs), particularly focusing on the connection between enterovirus (EV) infections and Type 1 Diabetes. Although there is a well-known link between these two, proving that one causes the other has been challenging. To tackle this, ENT1DEP is using a comprehensive strategy that combines various scientific disciplines and cutting-edge technology to delve into the underlying mechanisms and identify key biological indicators.
The project revolves around three critical questions:
- Why are insulin-producing β-cells the only ones destroyed by EVs? It’s suspected that these cells might have weaker antiviral defenses and more receptors that viruses use to enter, which could allow the virus to linger. To explore this idea, researchers are employing advanced human cell models, such as stem-cell-derived β/α-cells and organoids, as well as examining pancreas tissues from individuals with T1D.
- Why do only some people develop T1D after an EV infection? Some individuals might have less robust immune responses to the virus, leading to its spread to the pancreas, where it can cause persistent infections and inflammation that triggers diabetes. This theory is being tested by studying immune responses in children from birth to the development of T1D and investigating genetic factors that might influence virus persistence.
- How can we reduce the risk of EV-associated T1D? Researchers are looking into vaccines that could boost immunity to the virus and antiviral drugs that might eliminate persistent infections. They’re analyzing samples from the first trials of EV vaccines and T1D antiviral treatments to find biological markers that can predict the effectiveness of these interventions. They’re also testing whether vaccine-induced antibodies can prevent diabetes in mice and evaluating how antiviral treatments affect virus clearance and immune responses.
Project information
- Grant agreement ID: 101137457
- Start date: 01.01.2024
- End date: 31.12.2027
- Funding: € 7.144.790,00
- Coordinated by Tampere University
Objectives
- To identify why only β-cells are destroyed by EVs, while other pancreatic endocrine cells remain intact
- To determine why certain individuals develop T1D after EV infection while others do not
- To achieve optimal ways to attenuate EV-associated T1D risk
Expected results
- Identification of mechanism(s) rendering β-cells more susceptible to EV infections.
- Identification of individual susceptibility factors for EV infection, their persistence and development of T1D, and their correlation with similar susceptibility factors previously reported in other NCDs (e.g. long COVID).
- Biological proof-of-concept for prevention of EV-induced T1D by antibodies induced by EV vaccine in humans, assays for reliable distinction of vaccine- vs. infection-induced immune responses, markers predicting antiviral drug efficacy for T1D prevention.
The ultimate aim of ENT1DEP (Enterovirus-linked Type 1 Diabetes Exposed – mechanisms and Prevention) is to pinpoint individuals at high risk of developing T1D due to EV infections and intervene early. The findings from this project could also advance the prevention and treatment of other similar diseases, extending the benefits of this research. Moreover, this new knowledge is being shared with key stakeholders to enhance strategies for combating NCDs globally.
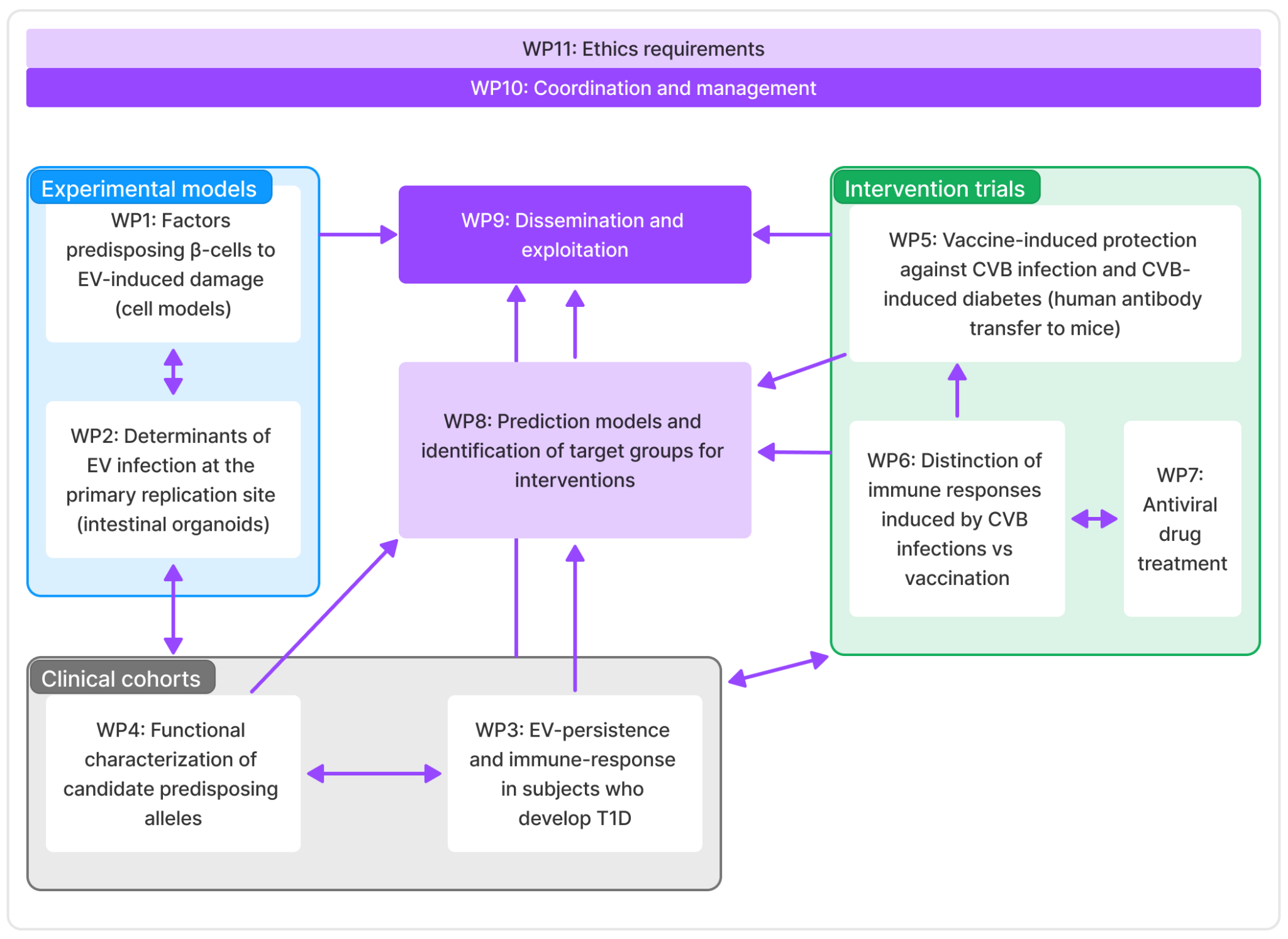
Work packages
- WP1: Factors predisposing β-cells to EV-induced damage, lead by University of Helsinki
- WP2: Determinants of EV infection at the primary replication site, lead by Tampere University
- WP3: EV-persistence and immune-response in subjects who develop T1D, lead by INSERM
- WP4: Functional characterization of candidate predisposing alleles, leady by Tampere University Hospital
- WP5: Vaccine-induced protection against CVB infection and CVB-induced diabetes, lead by Karolinska Institute
- WP6: Distinction of immune responses induced by CVB infections vs. vaccination
- WP7: Antiviral drug treatment, lead by Oslo University Hospital
- WP8: Prediction models and identification of target groups for interventions, lead by Tampere University
- WP9: Dissemination and exploitation, lead by empirica
- WP10: Coordination and management, lead by Tampere University
- WP11: Ethics requirements, lead by Tampere University